Professor Barcikowski, why does industry need nanoparticles?
Nanoparticles have applications in many different industries – and play a particularly significant role in the chemical industry. Here, they make a variety of products possible or economically viable in the first place and are used, for instance, in fuel cells, exhaust gas catalysis and 3D printing. Nanoparticles act as catalysts – as highly reactive coatings that trigger or accelerate chemical reactions.
Why are nanoparticles able to do this?
The greater the relative surface area of a material, the more it reacts – and nanoparticles have a huge surface area. Let me explain what I mean: if you cut a ball into ten pieces, you will have increased its surface area a hundredfold – i.e. it is enlarged exponentially with every cut. Eventually, you would end up with nanoparticles. Whenever the chemical industry needs platinum for catalysis, it buys it in the form of nanoparticles, because only a small amount of platinum is then required for a large platinum surface area.
Let’s talk about your manufacturing process. Am I right in thinking that you can produce indus-trial-grade gold nanoparticles with nothing more than a piece of gold, a glass of water and a laser?
To put it more generally, “a glass of liquid” and, to be more precise, a “pulsed laser” – but, yes, that’s all you need to make gold nanoparticles.
I'm assuming it's a bit more complicated than that.
Not really. Whether cutting, welding or drilling, all laser processes produce nanoparticles, which then evaporate as smoke. Some 15 years ago, during a research project on laser texturing, we used an airbrush nozzle to eject the smoke from a home improvement store in order to protect the electrical systems and the workpiece. We then noticed that the spray water changed color. This was due to the nanoparticles. Our idea was that we could extract the nanoparticles from the process and use them. This gradually led us to our current process. We place the target material – e.g. gold, platinum or silver – in liquid and guide an ultrashort pulse laser over it. Minute particles of the surface material evaporate within nanoseconds and accumulate within the liquid.
Why in liquid? Why not capture the nanoparticles from smoke?
Because nanoparticles are easier to capture – and handle – in liquid; here, we refer to “colloids.” Nanoparticles are highly reactive and immediately begin to clump together in air. In water, however, the pressure is greater – and this holds our particles in place within a lentil-like microbubble following laser treatment; within this microbubble, the particles cool at an incredible rate of 1,012 kelvins per second. The bubble eventually bursts, scattering the nanoparticles throughout the liquid, where they then float around at room temperature – without clumping together. This all happens within the space of microseconds. The second reason is more interesting: the properties of nanoparticles produced in water using lasers are even more special. For kinetic reasons, for instance, they have even more defects.
And that’s a good thing?
Absolutely! We want our nanoparticles to have as many defects as possible. The smaller you make a solid body, the more angular it will be. In the industry, we use the term “faceted.” The several thousand atoms in the particles separated by laser really want to arrange themselves in ideal facets, i.e. facets that fit cleanly together. But because they cool down so quickly and therefore solidify, they don’t manage it in time, meaning that the facets stay rough – like a cliff face. This is what we mean by “defects.” They activate the surface. And as I mentioned above, more surface area translates into more reactivity.
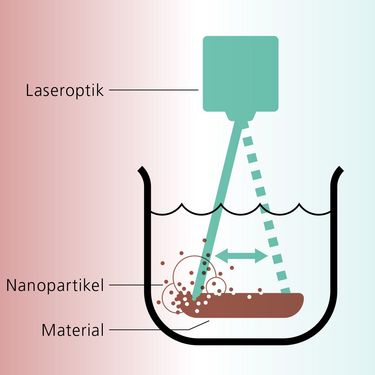
Nanoparticles comprise some thousand atoms/molecules or less. Owing to their special chemical properties, they are employed in various sectors, such as in medicine and in the chemical industry. (Graphic: Gernot Walter)
Up to now, the chemical industry has made its own nanoparticles. Why would they want yours?
Because mine are better. As I say, laser-generated nanoparticles are more reactive, i.e. more effective, on account of their defects. They also last longer, thus increasing the service life of a product. Just recently, two comparative studies on diesel catalytic converters and electric catalytic converters in fuel cells demonstrated that our nanoparticles perform better than traditional wet chemistry-based equivalents when it comes to eliminating nitrogen oxide within diesel catalytic converters. What’s more, our particles were much more stable, both in diesel and in the fuel cell. To be honest, we don’t yet know where this stability comes from, but we are working on it. It’s certainly a key factor for industry.
Is your laser-based process suitable for the mass production of nanoparticles?
That depends. We’re not yet thinking in terms of tons, but certainly in kilograms. For many applications, it’s not just about sheer volume. Instead, it is much more important to produce the particles in a simple and precise manner. Our laser-based process is, for instance, establishing itself in the field of large-scale industrial research. This is the first time that laser material processing is making an impact in the chemical industry – a market so huge that it’s hard to get your head around.
Research is always good, but is it already possible to earn money with your laser nanoparticles?
Yes. There are multiple approaches. In the last three years, we have boosted the productivity of the man-ufacturing process by a factor of 100. We believe that a further factor of 10 to 100 is realistic – and already have a few specific ideas. The process starts becoming economically viable at a rate of about half a gram of nanoparticles per hour. We are already at several grams per hour. These can then be mixed with the usual small mass fraction to create kilo packs of catalyst powder, which can be easily stored. But there are also other powders that we enhance using our nanoparticles.
Care to share?
Powder for 3D printing. We are currently working with a big-name steel manufacturer in this area. The aim is to round off metal 3D printing powder with a dash of nanoparticles and thus enhance the printing process. One laser process drives another.
What other benefits does the laser-based process offer?
Wet chemistry is clearly well ahead of us when it comes to volume. But there are certain things that we can do better. For many applications, industry requires nanoparticles made from alloys, rather than pure metals. This is where laser technology comes into its own. The laser-based process is perfect in terms of systematically producing ranges of different alloys with different mixing ratios; it could hardly be any faster. What’s more, the alloy nanoparticles come in the highest grade of purity, as we do not use any stabilizers or precursors (like those in wet chemistry that are present on the surface). As such, there is no need for laborious cleaning.
What can we expect next from your laboratory?
To put it in simple terms, a fully automatic “coffee machine” for nanoparticles. Two of my team members have already built a prototype and are setting up a company for it. The principle is simple: the laser machine is positioned on a desk; inside, it contains a replaceable cartridge with target materials. You press a button – “platinum,” “gold,” “silver” or whatever – and out come the desired nanoparticles. You don’t even have to know anything about nanoparticles to produce them. What’s more, the device is safe and reliable. All you need are an electricity supply and a bottle of water.
Nanoparticles for all?
Precisely. There is, after all, a considerable market for small quantities – and the process of ordering such day-to-day amounts is extremely annoying and unreliable. The fully automatic machine is just one example of the manifold possibilities of the laser-based process in liquids.
So would you recommend to an entrepreneur that they get involved in nanoparticle production?
Yes, why not? Nanoparticles are the future.

Stephan Barcikowski is Professor of Technical Chemistry at the University of Duisburg-Essen and researches the production and application of laser-generated nanoparticles. He and his team member Bilal Gökce are nominated for the 2020 Berthold Leibinger Innovationspreis in recognition of their pioneering achievements. Due to the coronavirus pandemic, the awarding of the prize has been postponed to 2021.