
Unique Device Identification (UDI) made easy
With the complete turnkey package from TRUMPF you can mark medical devices permanently and reliably using lasers and software from a single source. Increasingly medical technology products now need to be marked with a unique UDI code (UDI = Unique Device Identification) that are traceable across the entire supply chain. The most important requirement is for markings to be permanently legible, something that is frequently achievable only with laser marking.
What does Unique Device Identification (UDI) mean?
Unique Device Identification (UDI) refers to a global standard system for identification in medical products. A UDI marking consists of two parts – a machine-readable code and a human-readable code. The machine-readable part is represented either as a linear barcode or as a 2D Data Matrix Code, and the human-readable part with numbers and letters.
Patient safety is the top priority in medical technology. Clear and consistent medical-device labelling is fundamental to this - from manufacturers to specialist retailers and from hospitals to the patient. This maintains full transparency along the supply chain. Unique Device Identification enables those responsible for procurement and materials management to access regulatory specifications held in central databases. Serial numbers and expiry dates as examples of this. When a medical device is marked "UDI-compliant", no further details are required. In the foreseeable future, all medical devices in any risk class will require clear and machine-readable labelling.
All medical devices intended for sale in the United States or on the European market must have a UDI code. The goal is clear: a globally standardised UDI system. The medical technology industry in every market is being advised to label their products with UDI codes in anticipation of new legal requirements.
UDI labelling of medical devices is based on specific standards. To this end, the US Food and Drug Administration (FDA) has accredited the HIBC, GS1 and ISBT 128 standards. Generally labels always consist of two parts: the "DI" (Device Identifier) and the "PI" (Production Identifier). The DI, which is identical for each item, includes the ID of the manufacturer or labeller as well as the reference code of the product. In contrast, the PI is dynamic, containing information such as expiry and manufacturing dates the serial numbers. The global data exchange network GUDID enables manufacturers to forward product information to the American FDA UDI database.
In contrast to the US FDA regulation, the EU introduced a new identifier based on the European Union Medical Device Regulation (EU MDR) – "Basic UDI-DI". This enables medical devices with similar characteristics to be grouped in the EU approval database EUDAMED. The device can only be submitted to the authorities responsible for approval after the medical device manufacturer or an authorized representative has allocated the new identifier.
All information on US regulations is available on the website of the US authority, the US FDA.
All key European regulations are summarised on the EU MDR website.
All from one source: with marking lasers and software solutions from TRUMPF for correct UDI marking
TRUMPF has a broad range of marking lasers as well as customisable software solutions. Customers can use them to create UDIs from their own databases and apply the markings to medical products. Quality control and documentation functions are also available when required. TRUMPF customers get high quality markings from this complete package while also benefiting from TRUMPF solutions for UDI-compliant marking and process reliability.
Our passivation-resistant laser marking solutions ensure UDI conformity in 2020 and beyond.
Use a database connection to send DIs and PIs to TruTops Mark and automatically generate a UDI barcode for laser marking.
With our VisionLine Mark image processing, you can easily recognise, capture and verify UDI content.
Check the laser-marked content and perform individual quality ratings to reduce approval times.
TRUMPF is a recognized GS1 Solution Partner. GS1 is one of four authorities which develops and awards UDI codes as per accredited standards, in order to guarantee the traceability of medical products.
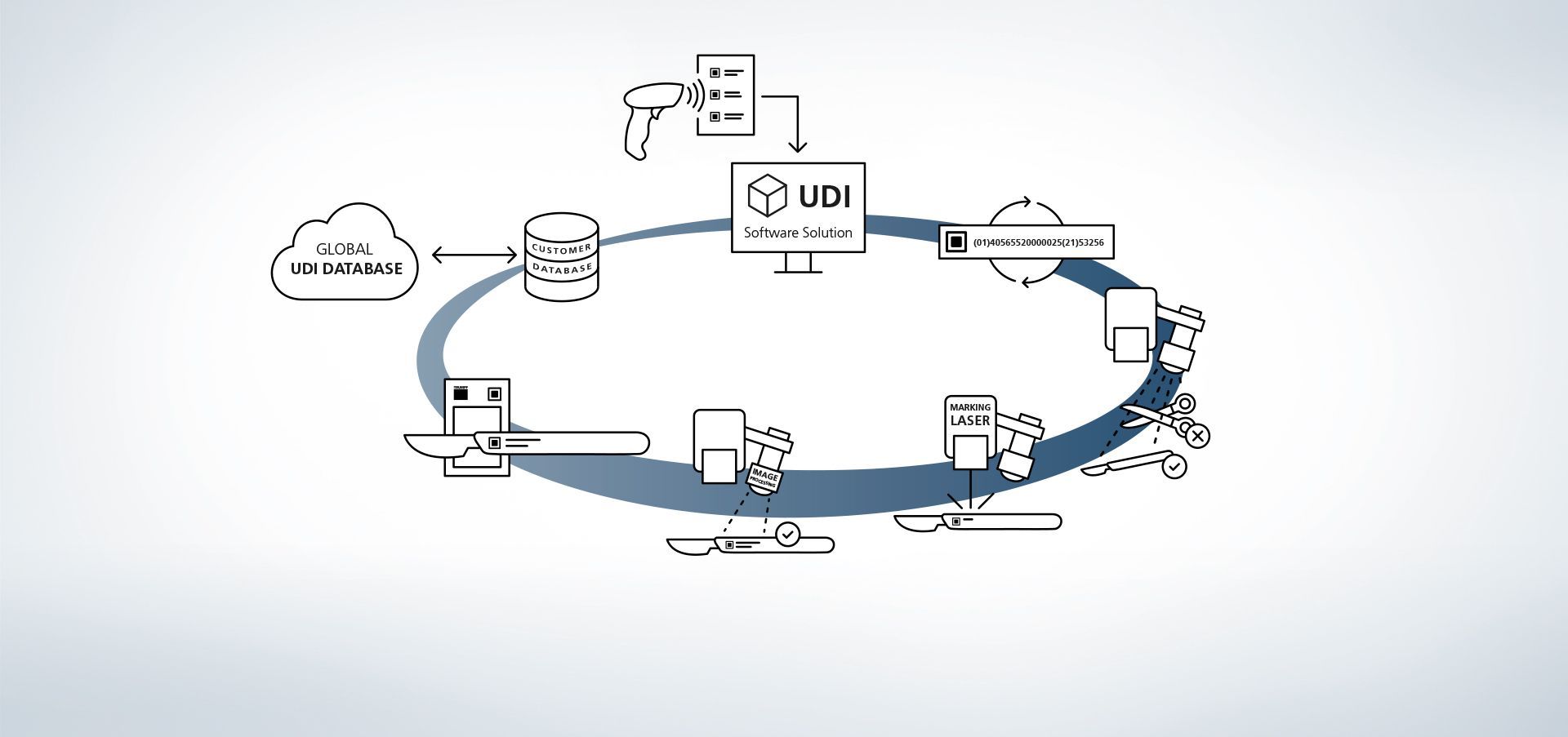
1. Access to database
The software is linked to databases. Reading information into the system via external hand scanners is also possible, for example.
2. Production data input
Data is available from the database or read out using a hand-held scanner.
3. Create UDI code
The UDI module creates a rule-compliant code from UDI-relevant data and individual extensions.
4. Component and position recognition
The image processing programs VisionLine Detect or VisionLine Model detect the component and its exact location and position. The software forwards the information to the controller which makes the marking in the precise position.
Find out more5. Marking laser
The TRUMPF laser marks the workpiece with a durable, corrosion-resistant and high-contrast marking that remains machine-readable and visible to the human eye even after numerous cleaning cycles.
6. Optional code content capture
With TRUMPF process sensors, even the end quality control is very easy. With the TRUMPF image processing solution, VisionLine, UDI-compliant codes can be recognised, read out and evaluated for quality using various methods.
Find out more7. Optional marking data check
Using the image processing programs VisionLine OCT (for character recognition) or VisionLine Code (for 1D/2D codes), marked data can be compared against the database and stored for documentation purposes with additional information if desired. This includes information on UDI code content and quality, or on the machines involved in the process. This ensures proper documentation of the component and marking even after many years.
Find out more



![[Translate to en_GB:]](/filestorage/TRUMPF_Master/_processed_/6/d/csm_Industry-MedTech-keyvisual_e61b5c909b.jpg)